How to participate?
Clinician
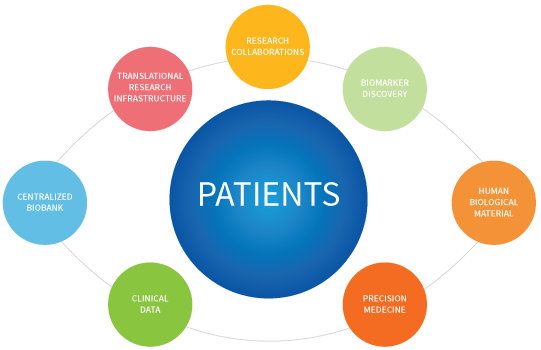
WHY SHOULD YOU COLLABORATE?
HOW TO COLLABORATE?
We are looking for investigators interested in recruiting cancer patients in the indications of interest for IMMUcan.
Patients will be recruited via SPECTA, the integrated research infrastructure under EORTC legal sponsorship. Once you become an authorized investigator within the platform, you will be able to recruit patients in all opened cohorts, including the IMMUcan’s ones.
Check here for more information about SPECTA.
Information on IMMUcan cohorts and patients’ eligibility criteria can be found from the SPECTA website.
Study sponsor
WHY SHOULD YOU COLLABORATE?

HOW TO COLLABORATE?
CHECK THE REQUIREMENTS FOR CLINICAL STUDY TO BE ELIGIBLE FOR IMMUcan
01
Academically sponsored study
Existing contractual obligation should not prevent the use of patient material for IMMUcan.
02
Adequate Patient
Informed Consent
It should authorize the use of patient material in collaboration with industry.
03
Clinical data availability
Clinical outcomes data should be available for IMMUcan not later than end of 2021.
Please check the full list of eligibility criteria
Researcher
WHY SHOULD YOU COLLABORATE?
HOW TO COLLABORATE?
Please check below the IMMUcan policy for access to data and Human Biological material.

INTERESTED TO COLLABORATE?
Please use the contact form. We will review your proposal and come back to you.

