IMMUcan
IMMUcan stands for “Integrated iMMUnoprofiling of large adaptive CANcer patient cohorts”
IMMUcan receives support from the European Union (grant 821558) within the Innovative Medicine Initiative 2 program and from the European Federation of Pharmaceutical Industries.
IMMUcan was started in March 2019 and will end in August 2025.
The IMMUcan consortium is composed by 18 academic organisations from European countries, 1 patient, 2 SME and
9 pharmaceutical companies. IMMUcan is under the leadership
of Merck and EORTC.
What will IMMUcan deliver?
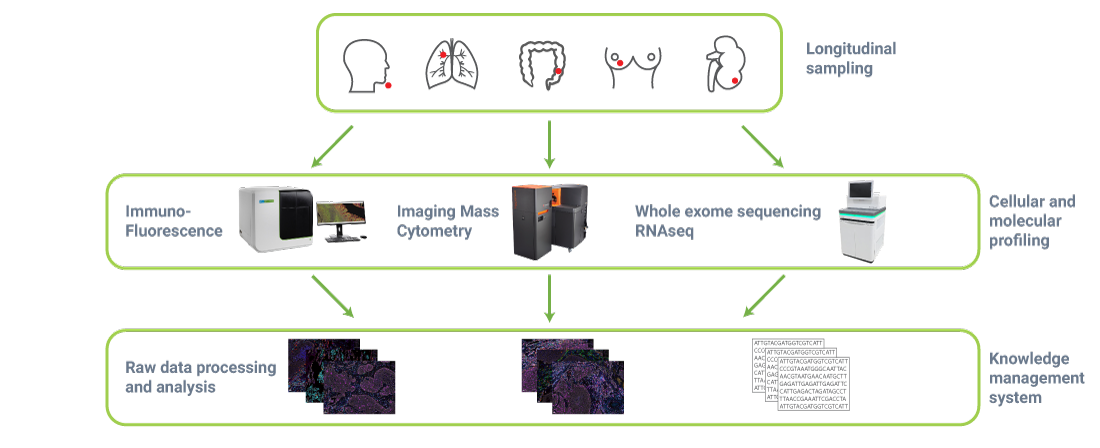
What is the project workflow?
What are the timelines?
How is the project organised?

WP1: Project management and coordination (Merck, EORTC, SIB, Bayer)
WP1 will support optimal project management in compliance with the highest scientific and ethical standards.

WP2: Communication, Public Relations, and involvement of Patient Advocacy Groups (EPF, EORTC)
WP2 will implement a dissemination plan covering scientific communication but also information to patients and lay people.

WP3 Legal aspects (EORTC, Merck)
EORTC has taken the legal responsibility for the collection and use of patients’ biological material and clinical data for IMMUcan. WP3 will manage legal aspects including access to project data and results by partners and 3rd parties.

WP4.1 Broad profiling (EORTC, Merck)
Genomic profiling using RNAseq & WES (CeGaT) and immune profiling multispectral analysis (CHUV) and tissue CyTOF analysis (UoZ) will be performed on all tumour samples.

WP4.2 Deep profiling (KUL, Merck, Servier).
Deep Profiling of a subset of patients will aim to characterize baseline and post treatment immune-suppressive tumour microenvironment features. WP4.2 will involve various techniques such as single cell RNAseq or liquid CyTOF.

WP5 Material, collection, processing and banking (IBBL, EORTC, Merck)
WP5 will be accountable for the collection of high quality biological samples and clinical data. IBBL is IMMUcan’s central biobank. EORTC provides access to patient material via the SPECTA program.

WP6 Biomarker validation (SIB, Abbvie)
WP6 provides a formal mechanism to prospectively evaluate proposed biomarker hypotheses, either those already formulated prior to IMMUcan or discovered from parts of IMMUcan data.

WP7 Data integration and bioinformatics (SIB, Sanofi)
WP7 will integrate and analyze the complex generated datasets using supervised and unsupervised approaches.

WP8 Database and IT infrastructure (SIB, Bayer)
WP8 will develop a dedicated knowledge management system to store and share data and results. A web portal and tools enabling access to raw and processed data will be set up.
